New & Noteworthy
That Sweet Spot of Mistranslation
August 03, 2017
Anyone at a party knows that a little alcohol can make you charming, but a lot can doom any relationship from blossoming (just listen to Drunk Uncle!). In fact, too much can destroy a party! You need to have enough alcohol to curb social inhibitions, but not so much you overwhelm them.

Just as drinking too much alcohol can be toxic to people, too much mistranslation can be toxic to a cell. from pbs.twimg
According to a new study out in GENETICS by Berg and coworkers, something similar sometimes seems to be true when the genetic code evolves. A variety of beasts, including the yeast Candida albicans, have slightly different genetic codes—one or a few codons code for a different amino acid than usual. This is often the result of a mutated tRNA, the molecule that carries the right amino acid to the right codon.
These authors found that mutating only the anticodon, the part of the tRNA that recognizes the codon, is not a great way to head down the path to a new genetic code. That mutated tRNA leads to too many of one amino acid being replaced with another. Like the boorish drunk who kills the party because he has had too much to drink, the cell is overwhelmed with too many of the wrong amino acids scattered across its proteins and dies.
What Berg and coworkers found was that having fewer of these tRNAs with a mutated anticodon allowed for a tolerable level of amino acid substitutions. This means that this tRNA can hang around until it is helpful, like when it can suppress a new mutation of a key amino acid in a key protein.
The authors dubbed these low level mutated tRNAs as “phenotypically ambivalent intermediate tRNAs”—tRNAs that are in the process of changing the genetic code for at least one codon. It may be that the variants of the genetic code found in nature arose this way.
They started out with a strain of Saccharomyces cerevisiae with a mutation that inserted a proline in the wrong place of the TTI2 protein. This strain does extremely poorly in the presence of 5% ethanol.
The authors then tried to create and/or isolate suppressor mutations in a serine tRNA that could allow the strain to grow in the presence of 5% alcohol. The idea is that this tRNA would now carry a serine to that troublesome proline codon.
They started off by changing the anticodon of a serine tRNA to UGG. Now, the cell would put a serine in at CCA proline codons.
When they transformed a plasmid carrying this tRNA into their yeast strain, they got very few colonies. This obnoxious tRNA overwhelmed the cell by changing too many prolines to serines. It ruined the party!
They next set out to find a way to bring this bad boy under control. They mutated the serine tRNA with the proline anticodon using UV mutagenesis and found four mutants that allowed this yeast strain to grow in 5% ethanol.
Each mutant tRNA had a single mutation: G9A, A20bG, C40T, and G26A. Berg and coworkers set out to figure out why the cells now tolerated the mistranslation they couldn’t handle before.
What they found was that at least for two of them, G9A and G26A, the cells dealt better with the mutated serine tRNA because there was less of them around. The toxic drunk had become the tipsy charmer!

I can teach you lots of things about biology but you’ll need to learn about drinking alcohol on your own. Beer mug from Max Pixel (http://maxpixel.freegreatpicture.com/).
Well, maybe not quite charming, but at least something that could be dealt with. Both mutated tRNAs affected cell growth in the absence of ethanol, with the G26A version having the more severe effect—a reduction in growth by 70%.
Most likely there was less of the G26A variant because it was a victim of the rapid tRNA decay (RTD) pathway. The G26A variant affected the growth rate much less in the absence of alcohol in a strain deleted for MET22, a key gene in the RTD pathway.
By looking at the crystal structure of a serine tRNA in complex with its aminoacyl tRNA synthetase from Thermus thermophilus, Berg and coworkers predicted that the G9A mutation should result in a poorly folded tRNA. They found this was indeed the case when they compared the melting curves of the G9A mutant and the tRNA lacking the G9A mutation.
So what we have are some tolerable, but by no means benign mutations. For example, the G26A is quickly selected against in the absence of ethanol.
This makes it hard to imagine how these sorts of mutations might arise and one day permanently alter a genetic code. The key to understanding how this might happen is a set of experiments Berg and coworkers did that showed that both G26A and G9A have little or no effect on cell growth in the absence of a mutated anticodon. In other words, tRNAs can exist in a poised state, ready to easily adapt with a single change to the anticodon if need be.
And it turns out that poised tRNAs may exist in the real world. For example, human tRNAs have a lot of variation. Perhaps these are around to one day save a cell with a mutation that would normally be deadly.
As this work (and real life) shows, too much of a good thing can be bad. This is true of alcohol (remember high school or college?) and true of some mutant tRNAs. Yeast can teach us about the tRNAs, the rest we need to learn on our own. #APOYG
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: MET22, mistranslation, tRNA, TTI2
Now Yeast Even Finds Fungal Pathogens!
July 27, 2017
Some products have lots and lots of uses. Sometimes they are designed that way like the hypothetical Shimmer, a floor wax and dessert topping from Saturday Night Live. And sometimes they can be used in more situations than they were originally designed for like Windex in the movie My Big Fat Greek Wedding, or the most versatile thing out there in real life, duct tape. (Click here and here for some fun duct tape uses).
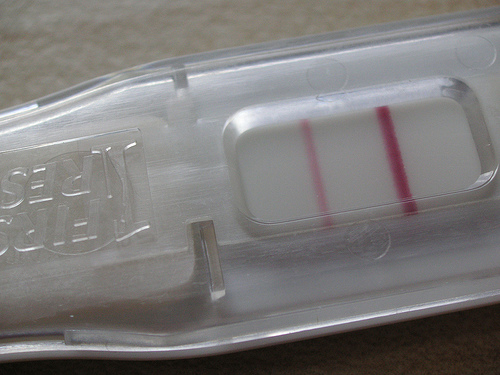
Yeast might one day be used to identify fungal pathogens with an assay as easy to use as a pregnancy test. from Wikimedia Commons
Wait, did I say duct tape was the most versatile thing out there? Maybe I spoke too soon.
Our friend Saccharomyces cerevisiae is in contention with duct tape for that title. Of course it is used to make bread, beer and wine, but it has lots of other uses too. Just a very few include using it to: make antimalarial drugs, understand how our cells and a surprising variety of human diseases work, understand how evolution can happen, understand how cancer forms and progresses, and make ambergris (whale puke). In the near future, it may even help deal with climate change by making biofuels out of agricultural waste, and pain management by making opioids.
And now a new study by Ostrov and coworkers adds another use—the simple and inexpensive detection of fungal pathogens. This is a big deal because if it works the way the authors think it will, this new use could one day save the lives of a significant fraction of the two million or so people who die from fungal pathogens each year.
In a nut shell, these authors have engineered S. cerevisiae to be attracted to different kinds of fungus by monkeying with its pheromone detection system.
S. cerevisiae comes in two mating types, a and α. The a-type cells make the secreted mating pheromone “a-factor” and STE2, a receptor that responds to “α-factor.” And α-type cells make the secreted mating pheromone “α-factor” and a receptor, STE3, that responds to the “a-factor.” So, thea-type cell makes something that makes it attractive to α-type cells and vice versa.
The end result of the factor/receptor interaction is that a number of genes are regulated so that the two haploid cells, a-type and α-type, can merge to become an a/α diploid. Fertilization yeast-style.
What these authors have done is to switch out the STE2 receptor from S. cerevisiae with the receptors from other fungal species in a-type cells so that the newly engineered cell responds to the presence of the new fungal species’ pheromone. For example, they replaced S. cerevisiae STE2 with the STE2 from Candida albicans so that the newly engineered cell now responds to pheromones from C. albicans.
Now of course that doesn’t make much of a sensor! No, they also need a readout to know that the engineered yeast has detected the presence of C. albicans.
They started out using a fluorescent reporter to see whether their engineered system responded to C. albicans mating pheromone. The cells fluoresced when the C. albicans mating pheromone was around at low levels but not with any of nine other fungal mating pheromones. So the system is definitely working—it can specifically identify C. albicans.
But to make it more useful in the developing countries where fungal pathogens are the biggest problem, they wanted to create a system with a more visible readout. For this they turned a bright red pigment called lycopene.
They needed to add three genes from Erwinia herbicola to get yeast to make lycopene: crtE, crtB, and crtI. They placed the first two under constitutive promoters (pADH1 for crtE and pTEF1 for crtB) and the third gene, crtI, under the control of the pheromone-inducible promoter from FUS1. So, lycopene is not made unless the appropriate pheromone is around to turn on the crtI gene.
This new system worked as well as the fluorescent one.
For the next step, they replaced the S. cerevisiae STE2 with STE2 genes from nine other fungal species involved in human disease and/or food or agricultural spoilage and showed that their system worked for all of them. These new pathogens included: Candida glabrata, Histoplasma capsulatum, Lodderomyces elongisporus, Botrytis cinerea, Fusarium graminearum, Magnaporthe oryzae, Zygosaccharomyces bailii, and Zygosaccharomyces rouxii.
And they didn’t stick to just the well-characterized fungal species either. They were also able to create a system that worked for Paracoccidioides brasiliensis, a less-well studied fungus that causes paracoccidioidomycosis (PCM), a disease endemic to Latin America.
It may be that just understanding a fungal species’ mating system is enough to create this sort of biosensor. More species will have to be tested to see just how universal the simple swapping out of a single gene will be.

Yeast is giving duct tape a run for its money in terms of versatility (although it can’t yet be used to make a hammock like this). From Ally’s Place (http://moonshadowangel.blogspot.com/2007/01/duct-tape-ho.html).
In their final step, Ostrov and coworkers tested whether they could create a simple, inexpensive dipstick test by spotting their engineered strains onto filter paper. For the initial test, they focused on two of their engineered strains: the one that detects the presence of C. albicans and the one that detects the presence of P. brasiliensis. They found that the system worked by simply putting the yeast-spotted filter paper into soil, urine, serum, and blood that had been supplemented with synthetic pheromone.
The yeast with C. albicans STE2 turned red with C. albicans pheromone, but not with P. brasiliensis pheromone and vice versa. And to increase its utility further, the authors found that the filter paper stayed functional even after being stored for 38 weeks at room temperature.
The impact of fungal pathogens on human health is understudied and underappreciated despite the toll they take on people’s lives, especially in the developing world. For example, as one recent review points out, “…at least as many, if not more, people die from the top 10 invasive fungal diseases than from tuberculosis or malaria.”
The basic research done on S. cerevisiae has allowed these scientists to devise an ingenious way to identify fungal pathogens. That magical combination of basic research and the most versatile eukaryote out there, S. cerevisiae, is poised to help humanity yet again. #APOYG
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: crtB, crtE, crtI, fungal pathogens, MATA, MATALPHA, mating, STE2, STE3
Call for Yeast Genetics Meeting 2018 Award Nominations!
July 25, 2017

The Yeast Genetics Meeting will be held August 22-26, 2018 at Stanford University.
You are invited to submit nominations to the meeting organizers for the awards and presentations that have become a cornerstone of the meeting.
- The Lifetime Achievement Award is given for lifetime contributions in the field of yeast genetics and outstanding community service.
- The Ira Herskowitz Award is given for outstanding contributions in the field of yeast research in the last 20 years. This award is usually given to scientists under 50.
- The Winge-Lindegren Address is a thought-provoking perspective given by a leader in the field of yeast genetics.
- The Lee Hartwell Lecture is given by a noted researcher in the field who has used yeast in a way that has had an obvious impact on other fields.
Previous awardees are listed on last year’s Yeast Genetics Meeting award site.
The deadline for nominations is Tuesday, August 1, 2017. Nominations should include: name, affiliation, email address, and a one or two sentence overview of why you are proposing the individual. Please send nominations to mahoney@genetics-gsa.org.
Categories: Announcements
Make Some More Room for Lamarck
July 17, 2017
For most people, a move to Tibet or other high altitude places is a real struggle. They suffer the many nasty symptoms of high altitude sickness while they are there.
Some people though, like natives of Tibet or of the Andes, have adapted to the extreme altitudes through natural selection and do just fine. How they adapted is a typical Darwinian story.

Lamarck’s theories are only mostly dead which means they are slightly alive. from the Brick In the Sky blog (https://thebrickinthesky.wordpress.com/).
Those who happened to have the right set of DNA did better than those who didn’t and so had more kids. Over time, the beneficial DNA became more common until the population was able to tolerate the low oxygen of the higher altitudes. (In Tibet, they may have acquired this helpful DNA by having kids with our close relatives the Denisovans.)
Imagine instead that the story went a bit differently. In a Lamarckian twist, let’s say that people who live in low oxygen were more likely to gain the traits needed to do well in this environment, strictly as a result of there not being enough oxygen around. In other words, the environment would make it more likely for the beneficial changes to happen.
Turns out this might have been the way the story went if people were more like yeast. And if dealing with low oxygen environments relied on a gene near a place in the DNA where replication forks often stalled. And if that the gene was upregulated in low oxygen.
It is under these conditions that Hull and coworkers found that yeast could preferentially develop the right mutations in an environment-dependent way. Instead of low oxygen though, these authors studied the yeast growing in high levels of copper.
One way that yeast deal with toxic levels of copper is to turn up the CUP1 gene. Or more precisely, turn up their tandem arrays of multiple copies of CUP1.
This last point is important because it hints at how yeast can increase the likelihood of beneficial mutations at CUP1 in the presence of copper. The increased transcription of the CUP1 genes increases the likelihood of a change in the copy number of these genes. Those yeast with increased copy number thrive in their new copper-tainted environment.
Now of course not every gene ends up with an increase in beneficial mutations just because it is induced. No, the gene also seems to have to be near where a DNA replication fork is more likely to stall. It is this combination of high rates of transcription and stalled DNA replication that can lead to changes in gene copy number.
The first thing the authors did was to map out where replication forks tend to stall in the yeast genome. These sites are marked by the presence of S139-phosphorylated histone H2A (γH2a).
Using chromatin immunoprecipitation sequencing (ChIP-seq) for γH2a they showed that likely stalling spots were within 1,000 base pairs of around 7% of the genes of Saccharomyces cerevisiae. These genes tend to be expressed at low levels under optimal conditions and higher levels under less ideal growth conditions. One of these genes is CUP1.
It is well known that when yeast are put into a high copper environment, the end result is yeast with more copies of the CUP1 gene. But this could simply represent the few cells that happened to have extra copies that then outgrow their compatriots with fewer copies. Ordinary old Darwinian selection.
What these authors wanted to show is that it is increased transcription that leads to increased copy number and not the selective pressure. They get at this a couple of different ways.
In the first approach, they introduce multiple copies of a Gal-inducible reporter at the CUP1 locus. In this system there is increased transcription in the presence of galactose, but no selection.
They found multiple colonies with changes in the copy number of the reporter gene with galactose and no differences in copy number with glucose. So, transcription matters.

Lamarck was only mostly wrong when it comes to evolution. Occasionally beasts can pass on traits they acquired over their lifetime. From Wikimedia Commons.
The second way they attacked this problem was by killing off any daughter cells to get rid of the problem of selection. In this strategy copy number mutants do not get a chance to outgrow their lower copy number sisters. Only the original mother cells remain.
Any increase in copy number would not be due to run of the mill Darwinian selection. Instead, they would be due to the presence of the factor in the environment the cells need to adapt to. And this is just what these authors saw happen.
They eliminated daughter cells using a mother-enrichment system in which daughter cells are killed in the presence of beta-estradiol. They treated a population of cells with beta-estradiol and then put half in normal media and half in media with 1 mM copper sulfate.
They found about a 9-fold increase in the number of copy number variants (CNV) in the presence of copper (27% CNV events compared to 3%). They did follow up experiments to show that copper was not acting as a mutagen—the copper had to induce transcription to cause the copy number variation. And judging by the bud scars, it looks like the cells divided more in the absence of copper, meaning this 9-fold increase is an underestimate.
So growing in the presence of copper increased mutations in the CUP1 gene that allowed the yeast to grow better in copper. Calling Doctor Lamarck!
We don’t have time to go into the rest of the experiments they did to flesh out their findings. For example, they show that this copy number variation can still happen even when they use tandem arrays that are much shorter than the usual 13 CUP1 copies. And that deletion of the H3K56 acetyltransferase RTT109 completely eliminates the effect of copper on the expansion of CUP1. And so much more! Anyone interested should definitely read the article.
These findings show us another example of Lamarckian selection. The environment itself causes the adaptations needed to prosper and these adaptations can be passed on. Not quite the ancestors of giraffes passing on their stretched necks to the next generation but still pretty cool.
The awesome power of yeast genetics again shows us something new about how life adapts and evolves. #APOYG
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: ChIP-seq, copper, copy number variants, CUP1-1, CUP1-2, Darwin, histone, Lamarck, replication fork, RTT109
How Histones Use FACT(s) to Find Their Way
July 05, 2017

The map is like FACT helping histones get to the right place. from publicdomainpictures.net.
Some people (like me) have no sense of direction. Send me to the store and who knows where I’ll end up!
Tools like maps, a GPS system, and my iPhone all help to make sure I get to where I need to be. And seat belts, airbags and working brakes keep me safe while I am getting there.
Histones are similar. These proteins, which help to organize and run our DNA, can get lost without a variety of helpers to show them the way. They also need to be kept safe as they travel.
Instead of an iPhone, histones get to where they need to go with the help of histone chaperones like Nap1p and the FACT complex. A new study by Hodges and coworkers in GENETICS helps to figure out which parts of histones interact with the FACT complex and, to a lesser extent, Nap1p.
Turns out that a few residues in an acidic patch on H2A/H2B dimers are critical for interacting with FACT. This makes sense given that previous work had shown that this acidic patch interacts with other proteins (although no one had shown it interacts with the FACT complex or Nap1p). Hodges and coworkers also identified other residues outside of the acidic patch that were important for FACT complex binding.
The first step was to analyze residues in this patch known to be lethal when mutated to alanine—H2A: Y58, E62, D91, and H2B: L109. The authors used co-immunoprecipitation (co-IP) assays against either Nap1p or Spt16p (a subunit of the FACT complex) to identify which, if any of these essential residues, was important for interacting with these histone chaperones.
As expected, wild type Nap1p and Spt16p interacted with both H2A and H2B in their assay. Of all of the essential residues of the acidic patch, only L109 on H2B significantly affected H2B’s ability to interact with the FACT complex. There was about a 4-fold decrease in the amount of Spt16p brought down with H2B: L109A compared to wild-type H2B in these experiments.
The authors decided to broaden their search for residues important for interactions by looking at those in the acidic patch that were not lethal when mutated to alanine. Recent work suggested two other residues, H2A: E57 and H2A: E93, might be important for getting the FACT complex to actively transcribed genes in yeast. Hodges and coworkers were able to confirm the importance of H2A: E57 in their co-IP experiments.
They now expanded to other residues on H2A and H2B that have been shown or hypothesized to be important for binding to the FACT complex—H2A: R78A, and H2B: Y45A, M62E. These authors found that only H2B: M62E significantly impacted binding to the FACT complex (H2B: Y45A had a small effect). They also found that H2A: R78A affected binding to Nap1p, but not the FACT complex.

Histones need to be shepherded to the genome by histone chaperones. by Mclaire MClaire, Wikimedia Commons
OK, so there is good co-IP data that H2A: E57, H2B: L109A, and H2B: M62E each affect binding of the FACT complex to the H2A/H2B dimer. These authors also provide good evidence that these mutants affect nucleosome occupancy and have nucleosome-based effects on transcription as well.
They used chromosomal immunoprecipitation linked to qPCR (ChIP-qPCR) against H2A and H2B to show decreased occupancy of H2A and H2B with the H2B: L109A mutant at four different promoters. Occupancy was around 3-4 fold lower than with wild type H2B.
They were also able to show that some of their H2A and H2B mutations mimicked the effects of partial loss of function mutations in the Spt16p part of the FACT complex. For example, just like mutations in SPT16 make a cell more sensitive to hydroxyurea, so too do H2B: Y45A, H2B: M62E, and H2A: E57A. These three mutants also induce cryptic transcription from the FLO8 gene like an Spt16 mutant.
Key residues in the acidic patch of H2A/H2B are critical for making sure histones get to the right place in the genome. Mutating them is similar to me not hearing the direction I need to go from my iPhone. Just like a histone not able to hang onto its chaperone, I will end up at the wrong place and not able to do what I needed to do.
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: FACT complex, H2A, H2B, histone, map, NAP1
Yeast’s Skynet Against Salt
June 27, 2017

Like Skynet, PI3,5P2 signaling is a rapid defense response. from Wikipedia.
In the Terminator franchise, the U.S. creates an artificial intelligence (AI)-based defense system called Skynet to, among other things, react more quickly to threats than any general or politician could. What starts out as an interesting idea almost dooms mankind to extinction once Skynet becomes conscious and decides to eliminate its greatest threat—humans.
Our friend Saccharomyces cerevisiae has its own version of Skynet for when it is “attacked” by too many salt ions. No, the system isn’t conscious and it does not threaten this yeast’s very existence but like Skynet, it is designed to react more quickly than more conventional systems based on gene regulation. It basically buys yeast enough time to allow the cells to more stably adapt to their new high salt environment.
Within 1 -5 minutes of being plunked down into high salt, yeast activates Hog1p, a key MAP kinase. The activated Hog1p heads into the nucleus and within 30-60 minutes, it tweaks the expression of a bunch of genes so the yeast can now better deal with its new environment.
This is a lot of time to be languishing in high salt. Luckily, yeast’s “whole hog” approach to high salt is not limited to just Hog1p. According to a recent study by Jin and coworkers in the Journal of Cell Biology, there is another, faster reaction to the high salt. And at least in these experiments, it is critical for yeast’s survival when it is assaulted by too much salt.
This rapid response involves a signaling lipid found in the vacuole called phosphatidylinositol 3,5-bisphosphate, or PI3,5P2. The amount of this lipid goes way up within just five minutes of the high salt shock.

One way to give Sarah Connor an easier life might have been to make Skynet’s control of defense transient. from cdn.movieweb.com.
PI3,5P2 is synthesized in yeast by a single enzyme, Fab1p. It stands to reason that if PI3,5P2 is critical to yeast survival in high salt, then deleting FAB1 should affect yeast’s ability to deal with all of those extra ions in its environment. This is just what Jin and coworkers found.
They compared the viability of wild type, hog1Δ, and fab1Δ strains under normal conditions and after a four hour exposure to 0.9M NaCl (high salt). Under low salt conditions, the fab1Δ strain was less viable than the other two. Around 30% of the fab1Δ yeast were dead.
At high salt, less than 10% of the wild type yeast and around 30% of the hog1Δ yeast were dead after four hours. This compares to the greater than 80% dead fab1Δ yeast.
The next steps in the study were to identify how the high salt increases the amount PI3,5P2. They reasoned that they needed something fast and that kinases just might fit the bill, so they started looking for strains that dealt poorly with high salt in the “…knockout haploid yeast mutant collection of 103 nonessential protein kinases.” They found a likely candidate in Pho85p and further work showed that its partner cyclin Pho80p was also involved.
Both the pho85Δ and pho80Δ strains had enlarged vacuoles (a common phenotype in yeast that cannot make PI3,5P2). More importantly, both strains could not make PI3,5P2 either under normal or high salt conditions and were also less viable than wild type under high salt conditions.
Additional experiments provided strong evidence that Pho85p phosphorylated Fab1p and that this phosphorylated Fab1p was important for synthesizing PI3,5P2 under high salt conditions. The final experiments confirmed that something similar happens in mammalian cells.
Jin and coworkers showed that the Pho80p-Pho85p equivalent in mammalian cells, CDK5-p35, phosphorylates the Fab1p equivalent, PIKfyve, in vitro. They also showed that CDK5-p35 is important for mouse fibroblasts to make more PI3,5P2 when exposed to high salt.
These studies suggest that yeast and probably mammals have at least two systems for dealing with high salt. The first is a rapid increase in PI3,5P2 that protects the cells from the environmental insult which gives the cells time to set up the second system—a longer term, more stable adaptation.
If only the folks in the Terminator world were as smart as yeast and had made Skynet a transient system set up to protect the U.S. while humans had time to respond in a more stable way. Think how much easier Sarah Connor’s life would have been!
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: 5-bisphosphate, CDK-P35, FAB1, high salt, HOG1, PHO80, PHO85, phosphatidylinositol 3, PIKfyve, Terminator
Network Maintenance at SGD on July 6, 2017
June 15, 2017

The SGD website (www.yeastgenome.org) and several of its resources will be unavailable on Thursday, July 6, 2017 from 7:00 am to 5:00 pm PDT (10:00 am to 8:00 pm EDT; 2:00 pm to 11:00 pm UTC) for electrical equipment maintenance.
During this brief maintenance period, the main SGD website (www.yeastgenome.org) will be unavailable for use. Other resources affected by the maintenance are listed as follows:
| Unavailable | Available |
| Downloads page | Genome Browser |
| SPELL | YeastMine |
| Textpresso | YeastPathways |
| SGD Wiki | YeastGFP |
We will make every effort to minimize any downtime associated with this maintenance. We apologize for any inconvenience this may cause, and thank you for your patience and understanding.
Categories: Maintenance
Making the Best of a Sticky Situation
June 05, 2017

Like turning lemons into lemonade, Hope and coworkers turned gummy yeast into a useful strain. from Pixabay.
Back in 1915, writer Elbert Hubbard coined the phrase, “When life gives you lemons make lemonade.” (His actual quote was “He picked up the lemons that Fate had sent him and started a lemonade-stand.”)
The idea of course is to take something bad and make it into something good. Like, if your research gives you terribly weak glue, invent Post-It notes.
Or as Hope and coworkers show in a new study in GENETICS, when your yeast experiment gets gummed up because the yeast evolves a sticky trait, do additional experiments to learn about the evolution of that complex trait. And “invent” a way to make the yeast less likely to flocculate, or stick together.
This new “invention” will be very useful for anyone trying to evolve yeast to create new products or to study how evolution works. It also showed that for this trait, under these conditions with this strain, there was one major way to get to flocculence—up-regulating the FLO1 gene. This meant they could greatly reduce the risk of this trait popping up by simply deleting FLO1.
Studying evolution in yeast often involves using a chemostat, an automated way to keep the yeast growing through repeated dilutions with fresh media. Scientists use this method to study how yeast evolves under varying conditions over hundreds of generations.
An unfortunate side effect of this method is that it also tends to select for yeast that stick together. These yeast are diluted away less, often meaning they become more common over the generations.
In this study, Hope and coworkers ran 96 chemostats under three different conditions for 300 generations and found that in 34.7% of the cultures, the yeast ended up aggregated. This was even though they used a strain of S288c in which the FLO8 gene was mutated. This strain flocculates less often than wild type!
These authors picked the 23 most aggregated cultures to study in more detail. They found that 2 out of 23 strains aggregated because of a mother/daughter separation defect. The rest were more run-of-the-mill flocculent strains.
They next used whole genome sequencing to try to identify which genes when mutated caused flocculence in the strains. They saw no FLO8 revertants.
The two strains with a mother/daughter separation defect both had mutations in the ACE2 gene, which encodes an important transcription factor for septation as well as other processes.
By CBS Television (eBay item photo front photo back) [Public domain], via Wikimedia Commons.  By woodleywonderworks, via Flickr
By woodleywonderworks, via Flickr
No, these Flos don’t cause flocculence, FLO1 does.
FLO1-related mutations dominated the other 21. They found a couple of different Ty insertions in the promoter region of FLO1 in 12 of the strains and mutations in TUP1 in 5 more. Tup1p is a general repressor known to repress FLO1, so it looks like up-regulating FLO1 leads to flocculent cultures. Other candidate mutations were found in FLO9 and ROX3.
They wanted to try to identify the responsible gene(s) in strains where there was no obvious candidate gene and also to confirm that the genes they identified really caused the trait, so they next did backcrosses between each mutant strain and a wild type strain.
The backcrosses identified two other ways to get flocculence— by mutating either CSE2 or MIT1. The researchers also confirmed that the mutations they found, including these two new ones, were probably the main cause of the flocculence in each individual strain. This trait co-segregated with the appropriate mutation in a 2:2 pattern for 20/21 strains as is predicted for a single causal mutation.
The results are even more FLO1-heavy than they appear. Further studies showed that ROX3, CSE2, and MIT1 all require a functional FLO1 to see their effects.
So FLO1 appears to be the main route to flocculence in this strain. And the next set of experiments confirmed this.
Hope and coworkers ran 32 wild type and 32 FLO1 knockout strains in separate chemostats for 250 generations and found that 8 wild-type strains flocculated while only 1 FLO1 knockout strain did. Knocking out FLO1 seems to make for a more well-behaved yeast (at least in terms of evolving a flocculent trait in a chemostat).
And the strain can probably be improved upon even more. For example, researchers may want to limit Ty mobility as this was a major way that FLO1 was up-regulated, increase the copy number of key repressors or link those repressors to essential genes. Another possibility is to also mutate FLO9 as its up-regulation was the cause of that one aggregated strain in the FLO1 knockout experiment.
Researchers no longer need to “settle” (subtle, huh?) for big parts of their experiments being hampered by gummy yeast. Hope and coworkers have created a strain that is less likely to flocculate by simply knocking out FLO1. Not as ubiquitously useful as a Post-It note but a potential Godsend for scientists using yeast to understand evolution.
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: ace2, CSE2, evolution, FLO1, FLO8, FLO9, flocculation, MIT1, ROX3, TUP1
Creating an Ethanol-Making ‘Super Bowl’ Championship Team
May 24, 2017

Tom Brady makes a team better by making the players around him play better. The same thing can be said for a mutant RPB7 gene that makes other genes work together better as an ethanol-making team. By Paul Keleher [CC BY 2.0 (http://creativecommons.org/licenses/by/2.0)%5D, via Wikimedia Commons.
There are a few ways to turn a failing sports team around. One is to tailor individual training to make each player better. Now, the team is better overall because of the changes each player makes.
Another way to improve a team is to change a player in a key position who makes everyone better. A classic example of this is the American football team, the New England Patriots.
On September 23, 2001, Drew Bledsoe, then the starting star quarterback of the New England Patriots, took a savage hit from New York Jets linebacker Mo Lewis. The Patriots replaced Bledsoe with his backup, Tom Brady, and some might argue, the team (whom Brady led to their first Super Bowl win that year) and the NFL, has not been the same since.
Quarterback Tom Brady, along with head coach Bill Belichick, makes whomever the New England Patriots bring in better. Wide receivers, tight ends, and running backs can be replaced in the lineup without the team missing a beat. He just makes the players around him better than they might be on another team.
In a new study, Qiu and Jiang take a “Patriots” approach to ethanol production in the yeast Saccharomyces cerevisiae. Rather than improving individual genes on their own, these authors instead decided to “bring in” a new version of RPB7, a gene that encodes a key subunit of RNA polymerase II, the molecular machine responsible for making messenger RNA (mRNA).
They hoped that changing this pivotal transcriptional player would cause lots of other genes to do “better” so that “team” yeast would make a lot more ethanol. Their hopes were realized in their Tom Brady equivalent—a mutant they called M1. Yeast bearing this mutant RPB7 gene became the Super Bowl champs of ethanol production.
One of the keys to increasing ethanol production in yeast is to find strains that are more tolerant of high levels of ethanol. The more ethanol they can withstand, the more they can make.
These authors used error prone PCR mutagenesis of the RPB7 gene to find their game-changing mutant. They then took their library of ~108 clones and cultured them in increasing amounts of ethanol, selecting for more ethanol-resistant strains.
After 3-5 rounds of subculture, they plated the cells onto media containing ethanol. Around 30 colonies were picked and sequenced with the best mutant being the one with two mutations—Y25N and A76T. They named this mutant M1.
This mutant grew a bit better than the parental strain background, S288C, in the absence of ethanol, but where M1 really shined was when ethanol was around. It grew around twice as fast in 8% ethanol and could grow at 10%, a concentration that completely inhibited the parental strain from growing.
Being able to withstand high levels of ethanol is important, but it isn’t all that yeast have to deal with. There are multiple other stressors around when you are swimming in 20 proof media.
For example, yeast can suffer from high levels of reactive oxygen species (ROS). M1 not only tolerated 3.5 mM hydrogen peroxide, a proxy for ROS, better than the parental strain, but it also had around 37% of ROS levels inside cells than that of the parental strain. M1 can deal with high levels of ethanol and ROS.
The authors then tested how this mutant dealt with other potential fermentation problems. For example, acetate, a fermentation byproduct, and high levels of NaCl both inhibit yeast growth. M1 tolerated 80 mM acetic acid and 1.5 M NaCl better than the parental strain did.

A couple of mutations in the RPB7 gene makes yeast able to tolerate alcohol way better than this guy. By jerome Chua [CC BY 2.0 (http://creativecommons.org/licenses/by/2.0)%5D, via flickr.
M1 appeared to be a champion mutant for making ethanol, and the fermentation studies bore this out.
Under a wide variety of conditions, M1 outperformed the parental strain in terms of growth rate, cell mass, and amount of ethanol made. For example, after 54 hours, yeast containing the M1 mutation of RPB7 managed to make 122.85 g/L of ethanol, 96.58% of the theoretical yield. This is a 40% increase over the control strain. Quite the ethanol producer!
Finally, Qiu and Jiang used microarray analysis of the parental and M1 strains at high levels of ethanol to discover the genes that M1 affected. They found 369 out of a total of 6256 genes behaved differently between the two strains. Of the 369, 144 were up-regulated and 225 were down-regulated.
I don’t have time to go over all the genes they found but a great many of them make sense. As the authors write, “…a significant set of genes are associated with energy metabolism, including glycolysis, alcoholic fermentation, hexose transport, and NAD+ synthesis.” M1 seems fine-tuned for making ethanol.
A mutant subunit in RNA polymerase II has made yeast better at making high levels of ethanol, most likely by affecting many key genes at once. It is a fascinating way to quickly affect a whole suite of genes involved in a process. In the ethanol-making Super Bowl, we have a new champion yeast strain, M1.
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: ethanol, fermentation, NFL, Patriots, RNA polymerase II, RPB7, Super Bowl, Tom Brady
Mass Production in Yeast
May 11, 2017

This approach changed everything when it came to manufacturing in factories. Perhaps the ideas in this new study will change things for manufacturing in cells.Image from Wikimedia Commons
After Henry Ford invented the moving assembly line, manufacturing was never the same. With it, his workers were able to push out a car every 2 ½ hours instead of the 12 it used to take. (Another website said it was reduced to 90 min!) The technology quickly spread to every factory.
Now of course, an assembly line is only as fast as its slowest worker. If someone is taking extra time to bolt down that part, then everyone downstream will have to go slower too, resulting in fewer cars being made.
But, you also can’t go too fast. If you do, someone can get injured, shutting down the whole line. (Or the worker has to eat all the candy to keep up, like Lucy.)
And you want to make sure things happen in the right part of the factory. You don’t want the paint sprayer out in the open, poisoning factory workers. So, that needs to happen in a special room.
This also applies to cell processes where something complicated is built, step by enzymatic step. All the enzymes need to be at the right levels and in the right place to maximize the productivity of the whole process.
This all becomes very obvious when you try to move an enzymatic process from one beast to another. What worked perfectly before, now barely works at all.
One way to fix this is through trial and error, trying to optimize one part of the process at a time. This is incredibly time consuming!
In a new study out in Nature Communications, Awan and coworkers show one way to tweak all of the enzymatic steps involved in making penicillin at the same time in the yeast Saccharomyces cerevisiae. While this isn’t that useful for making this antibiotic (there are better ways available right now), it does show how researchers can apply the same techniques to perhaps identify and produce new antibiotics. And, it can also be applied to other unrelated enzymatic processes.
Penicillin is made in a five-step process in filamentous fungi. In the first part of the process, two enzymes create a tripeptide precursor using alpha-aminoadipic acid, cysteine, and valine, called ACV. This part of the process had been previously recapitulated in yeast, so Awan and coworkers used this as a starting point for their penicillin producing strain.
The next part of the process uses the last three enzymes and takes place in peroxisomes in filamentous fungi. These authors found that they only got penicillin when these enzymes were tagged to be sent to the peroxisome in yeast. Like a special room for spray painting cars, these enzymes need to be in the right place to make penicillin.
But this was by no stretch of the imagination an efficient penicillin-making machine. The thing managed only 90 pg/ml in the media. As Ursula from Little Mermaid might say, “Pathetic.”

From Tumblr
Still, it is a starting point. The next step is to get the yeast to crank out more penicillin. To do this, they used a combinatorial approach to optimize the process all at once. Well, not really all at once.
First, they set out to optimize how much of the precursor ACV the yeast made. Then, they optimized how much ACV was converted to penicillin.
Awan and coworkers created a library of low copy plasmids that had the genes for the first two enzymes, pcbAB and npgA, under the control of different pairs of promoters. One plasmid, with the pTDH3 promoter driving pcbAB expression, and the pPGK1 promoter driving npgA expression, outperformed all of the others. As measured by Liquid Chromatography-Mass Spectrometry (LCMS), the yield of ACV increased from 20 to ~280 ng/ml.
Next, the authors used this new strain as a starting point for optimizing the activity of the final three enzymes using a similar approach. They used a “…one-pot combinatorial DNA assembly using Golden Gate cloning…” to make a library of around 1000 high copy plasmids where each gene was under the control of one of ten different promoters of varying strength. Using LCMS they found strains that could make 3 ng/ml of penicillin, a significant improvement over the original 90 pg/ml.
The 3 ng/ml of penicillin in the media should be high enough concentration to inhibit the growth of bacteria like Streptococcus pyogenes. So, they confirmed that their penicillin was active using growth inhibition assays.
After sequencing the plasmids, the authors saw that the best strains tended to have strong constitutive promoters driving one of the genes, pclA, and medium strength promoters driving another one of the genes, pcbC. They used a minION DNA sequencer to confirm that this was not the result of a biased library.
As a final step, they set out to optimize penicillin production and to increase the throughput of their assay. They created another library that swapped six different promoters that varied in strength from medium to high for each of the last three genes in the pathway, pclA, pcbC and penDE. Instead of using LCMS to screen for penicillin production, they used a 96 well plate-based assay that looked for inhibition of Streptococcus pyogenes growth for their 120 new strains.
They selected 12 of the highest performing strains and confirmed by LCMS that they made lots of penicillin. Five of the strains made more than 5 ng/ml, a more than 50-fold increase over their original strain.
As this concentration is still three orders of magnitude below what other organisms can currently do, this new yeast strain will not go into penicillin production any time soon. But this study gives us a way to quickly optimize antibiotic production using growth inhibition assays instead of the more cumbersome LCMS.
And it isn’t restricted to just antibiotic production. Similar combinatorial approaches can be used for almost any stepwise enzymatic process. Researchers can create libraries of plasmids where levels of enzyme vary and use the long reads of minION DNA sequencing technology to confirm that their results are not skewed by a biased library.
As usual, this is only possible as a simple, easy procedure because of the awesome power of yeast genetics (#APOYG). Researchers have the tools to use yeast to find new antibiotics and to manufacture them at a high rate, like inventing the car and the assembly line at the same time.
by Barry Starr, Ph.D., Director of Outreach Activities, Stanford Genetics
Categories: Research Spotlight
Tags: ACV, liquid chromatography-mass spectrometry, minION, npgA, pcbAB, pcbC, pclA, penDE, penicillin synthesis